Both, the Medical Devices Directive 93/42/EEC (MDD) and Regulation (EU) 2017/745 on Medical Devices (MDR) require manufacturers of medical devices to draw up a clinical evaluation for their devices.
The overall aim of a clinical evaluation is to assess and analyze clinical data regarding the medical device to provide evidence for the product’s clinical safety and performance.
Important inputs for the clinical evaluation are provided by risk management, design inputs, instructions for use, clinical investigations, literature reviews and PMS etc.
Pre- and post-market clinical data that are relevant to the intended use of a device constitute the basis of the evaluation. Manufacturers may include data specific to the device under evaluation as well as data from equivalent devices (if equivalence may be claimed).
The output of the clinical evaluation is the clinical evaluation report. Clinical evaluation is a continuous process that follows the life-cycle of a device. With MEDDEV 2.7/1, rev. 4 and under the MDR, this process plays a central role in the CE Marking of medical devices. It is closely aligned with other important sections, e.g. Post-Market Surveillance (PMS) and Risk Management.
Key Steps
The overall approach should comprise several key steps as outlined below:
- Identification of regulatory requirements to be supported by clinical data
- Identification of available clinical data for the device under evaluation and state of the art for the intended use
- Determination if there is sufficient clinical data for the safety and performance of the device under evaluation
- If clinical data is not sufficient, generation of additional clinical data
- Determination of the device’s clinical safety and performance and benefit/ risk ratio
Process
Important inputs for the clinical evaluation are provided by risk management, design inputs and product specifications, labeling or instructions for use respectively, pre-clinical studies, clinical investigations, literature reviews and PMS. The outputs of the clinical evaluation, i.e. the clinical data on safety and performance then feed back into the risk/ benefit analysis.
The guidance document MEDDEV 2.7/1, rev. 4 gives a schematic overview of the different stages of the clinical evaluation process:
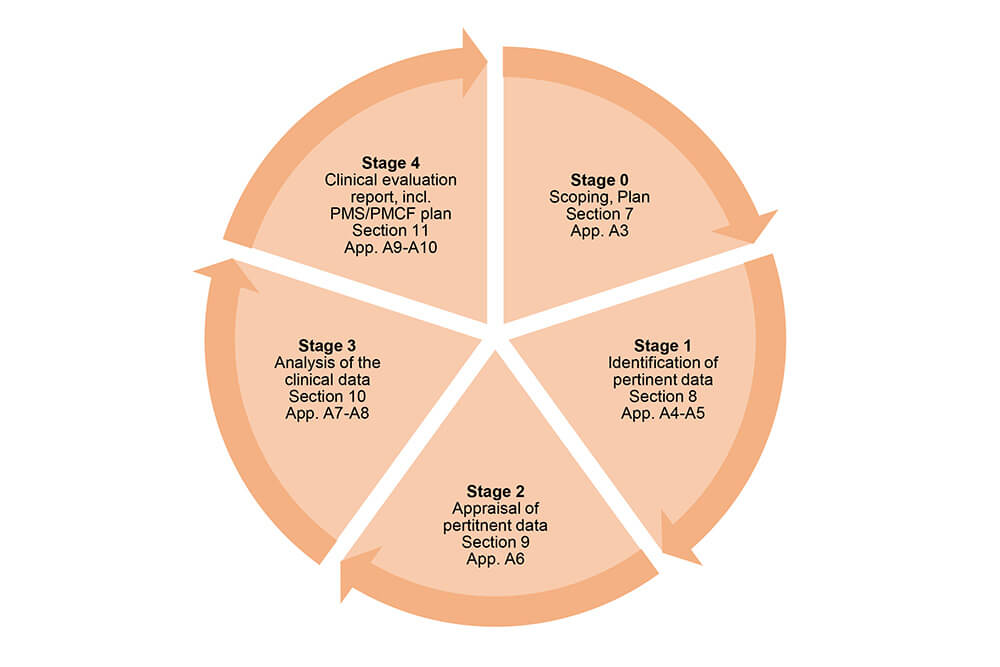
Source: MEDDEV 2.7/1, rev. 4
These have been largely adopted by the MDR and may be used as a reference until further guidance is available.
Sources: MEDDEV 2.7/1, rev. 4, MDR
For more information about clinical evaluations please read our related topics as linked below
Check our download section of Guidance Documents for the MDR.
Visit our Supportive Information Section regarding MDR / IVDR.
Or get in contact with our team.




