Till date, neither the European Commission nor a related organization has issued an official guidance document on the medical devices regulation (MDR) and in vitro diagnostic medical devices regulation (IVDR) post-market surveillance (PMS) requirements. ISO/ TR 20416:2020 provides valuable assistance in this context.
Although standards like ISO 13485 and ISO 14971 include requirements on PMS, they do not focus entirely on this process. The International Organization for Standardization has issued ISO/ TR 20416:2020 to close this gap: “The intent of this document is to provide guidance to manufacturers who are planning and executing their post-market surveillance activities” and should be read in conjunction with ISO 13485 and ISO 14971. (ISO/ TR 20416:2020)
Figure 2 schematizes the interplay between these standards and the technical report.
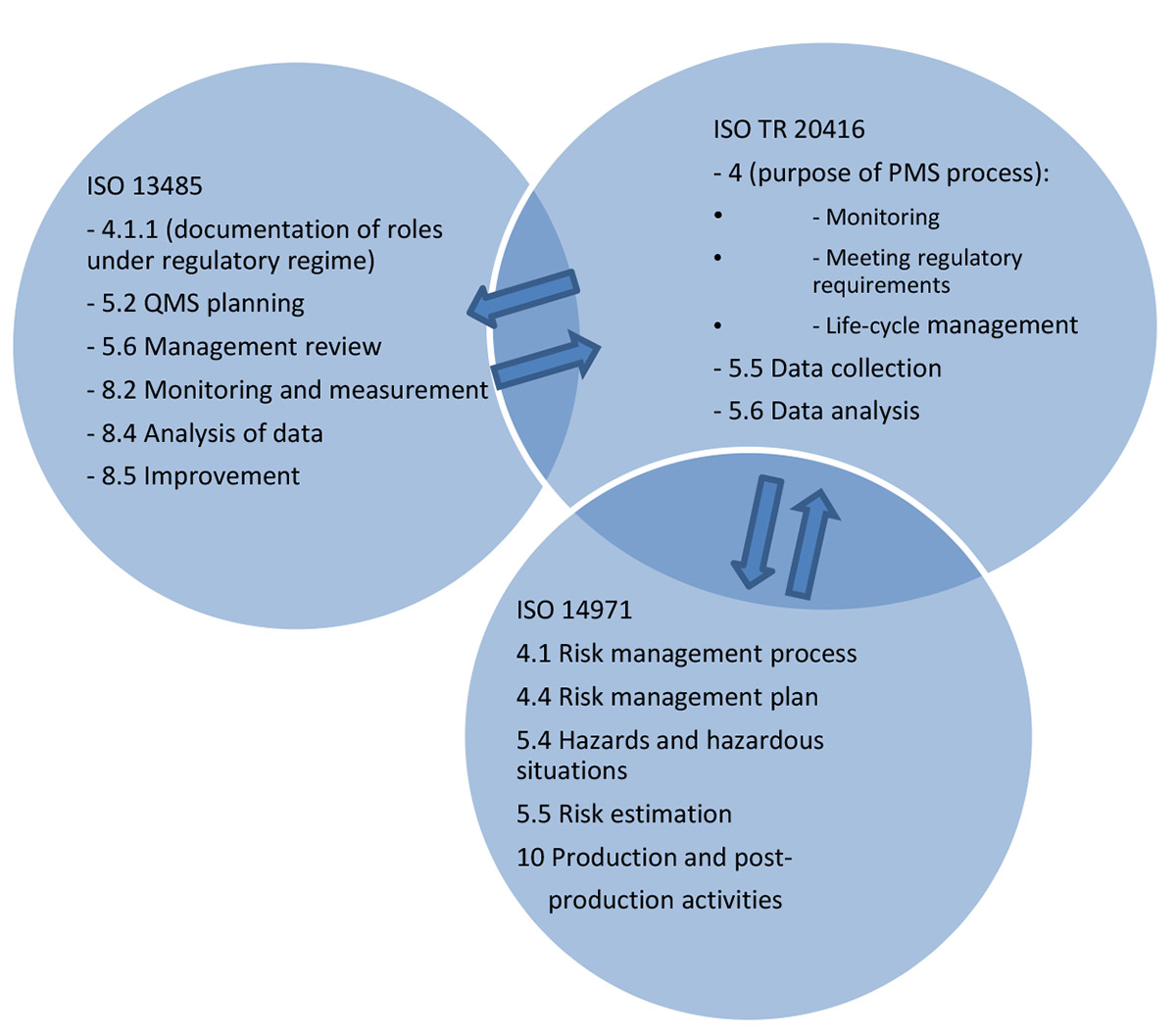
Figure 2. Interplay between ISO 13485, ISO 14971 and ISO TR 20416 (ISO/ TR 20416:2020)
ISO/ TR 2016:2020 provides insights how a manufacturer should build the scope and objective of the PMS Plan. More specifically, it should consider aspects like the applicable regulatory requirements, the classification, intended use and also what questions should be asked to establish the PMS Plan objectives. (International Organization for Standardization, 2020) The document contains a collection of potential data sources that a medical device producer may use for the PMS planning, and it also helps to specify the corresponding collection methods.
What seems particularly relevant is the guidance on the analysis of data. ISO/ TR 20416:2020 suggests qualitative and/ or quantitative methods depending on the data sources and PMS Plan objectives. The output of the previous steps will then be the PMS Report referring to the items previously defined in the PMS Plan. Lastly, the technical report provides practical guidance by giving further examples of data analysis methods and also by making available PMS Plan samples in the corresponding annexes.
To summarize, ISO/ TR 20416:2020 represents a valuable tool to implement some essential aspects of the MDR and IVDR PMS requirements
To summarize, ISO/ TR 20416:2020 represents a valuable tool to implement some essential aspects of the MDR and IVDR PMS requirements. Yet, the document does not cover all applicable requirements. For example, there is no guidance how to connect PMS to related processes like risk management or clinical evaluation or the QMS in general. In addition, the items below, which are required in an MDR and IVDR compliant PMS Plan, have been considered only briefly or at all:
- Methods and protocols to communicate effectively with competent authorities, notified bodies, economic operators and users
- Systematic procedures to identify and initiate appropriate measures including corrective actions
- Effective tools to trace and identify devices for which corrective actions might be necessary; and
- A PMCF/ PMPF plan
Source: ISO/ TR 20416:2020





